After 20 years of research, Genetic Tools Europe has developed a test method capable of detecting cancer cells significantly earlier than ever before, up to one thousand times more sensitive than current methods.
Who is this for?
A cancer diagnosis is one of the most difficult messages anyone can receive. It impacts not just the individual, but families, friends, and entire communities. While healthcare professionals work tirelessly to treat patients, the key to better outcomes often lies in early detection.
Cancer care can broadly be divided into two categories:
- 1
Detection: Population screenings, mammograms, scans, and PCR tests aimed at finding cancer as early as possible.
- 2
Treatment: The development of medicines and patient care strategies.
Genetic Tools Europe focuses on the first category. We use advanced DNA information to detect cancer at its earliest possible stage, long before traditional symptoms appear.
The Current Challenge
Many "improvements" in this sector are often marginal or based on wishful thinking. Our approach is different.
How It Works
Our breakthrough lies in a unique double-acting enzyme developed over decades of research, refining the established PCR method to unprecedented levels of sensitivity.
Ultra-High Sensitivity
While current methods detect roughly 1 in 100 cancer cells, our method detects 1 in 100,000 cells without false positives. This ultra-early detection allows for intervention before symptoms even appear.
Rapid Results
Speed is crucial. Our test delivers clear results within several hours. This efficiency reduces uncertainty and waiting times for patients and doctors alike.
Proven Foundation
Based on the trusted PCR technique and refined with our proprietary double-acting enzyme, this isn't just theory. It's a validated evolution of established diagnostic science.
The Breast Cancer Example
Currently, breast cancer is often found 5-9 years after onset via mammography or physical checks. Our method can likely detect gene mutations much earlier, in stage 0. This shift from "late detection" to "ultra-early detection" could fundamentally change treatment options.
A clear overview
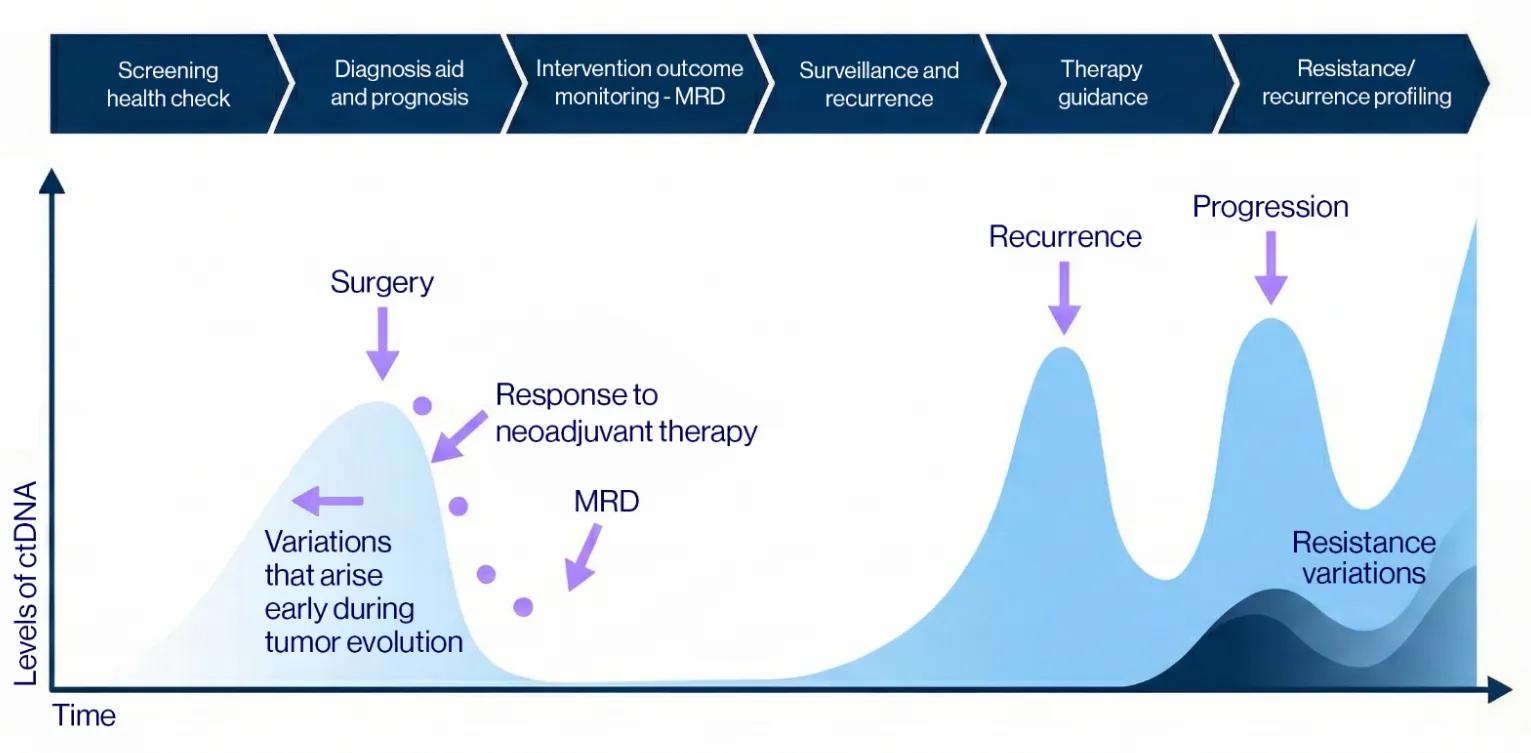
Our visual map demonstrates the power of tracking circulating tumor DNA (ctDNA) over time. From early screening and diagnosis to post-surgery monitoring, this technology detects microscopic traces of cancer (MRD) that standard tools may miss. By continuously monitoring ctDNA levels, we can identify recurrence early and understand if a tumor is becoming resistant to treatment, enabling timely and precise medical intervention.
Strategic Collaboration
Bridging the gap between cutting-edge science and public healthcare is a challenge. We are proud to have found a receptive and decisive partner in the NKI (Netherlands Cancer Institute / Antoni van Leeuwenhoek).
Recognized globally for their leadership in oncology, the NKI has validated our test method and potentials. Together, we have established and recorded the results that pave the way for this new standard in diagnostics.
Seamless Integration
Our method uses existing laboratory equipment found primarily everywhere. No new hardware, no complex new infrastructure. It fits seamlessly into the current healthcare workflow.
Global Validation
We have worked tirelessly to bring this innovation to the attention of scientists worldwide. The recognition by a top-tier institute like NKI confirms the scientific solidity of our approach.
Turning Cancer into a Chronic Disease
Our mission is bold but achievable: to detect cancer so early that it can be treated before symptoms arise, transforming it from a lethal threat into a manageable condition.
Future Outlook
We are currently formalizing test data with leading scientists to make this the new standard.
We are also finalizing production-ready mutations for Lung Cancer and Colon Cancer. This isn't just theory—it's practice in motion.
Active Development
Scaling for widespread application
Watch Our Story
A short film for everyone dealing with or affected by cancer.